Profile
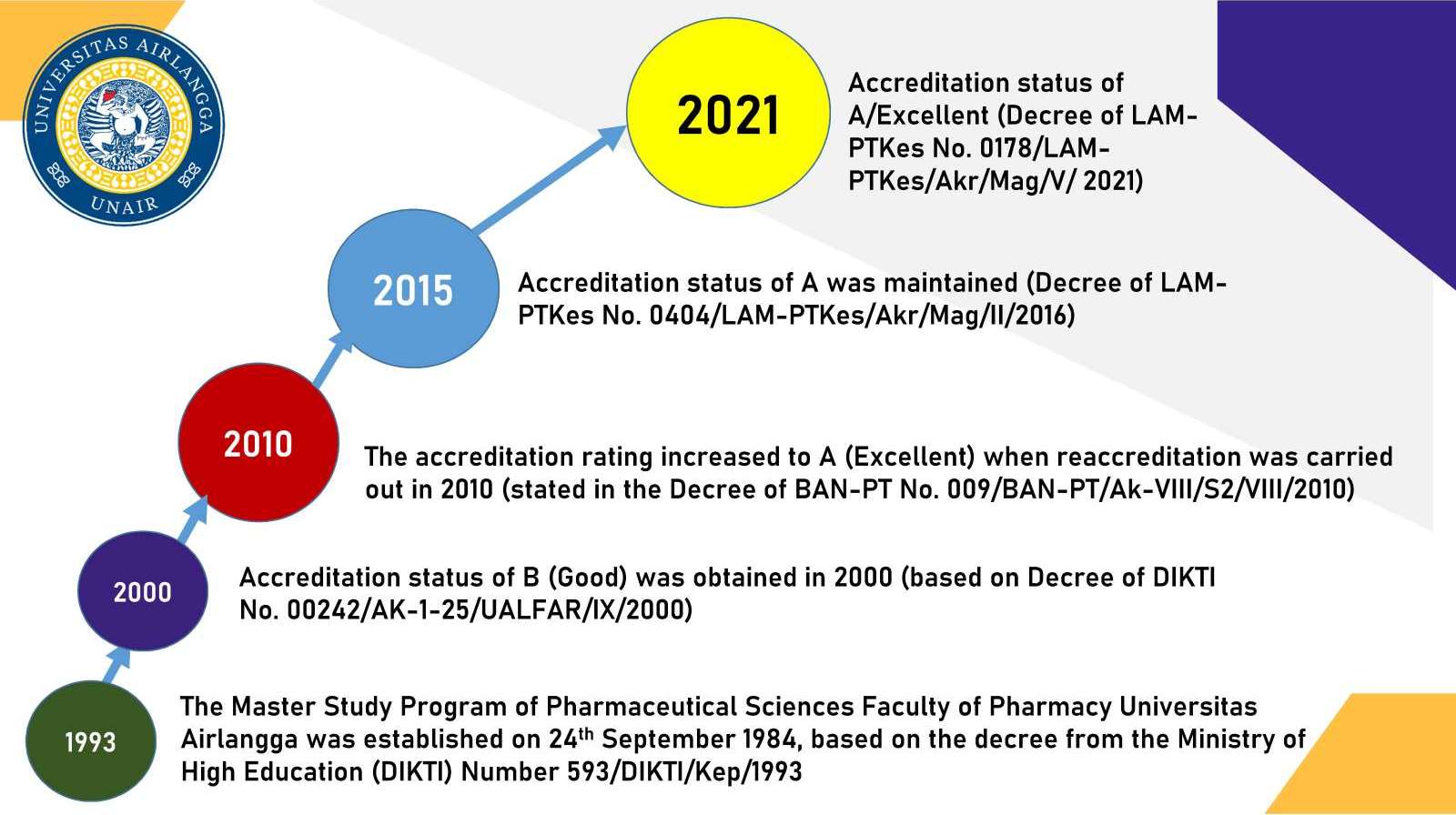
One indicator of the progress of a nation is the number and quality of human resources. The high number of university graduates, especially those with Master's degrees, means the ability of a nation to produce inventions, innovations and developments in science and technology, as well as its application to solve various national difficulties. This study program has been established since 1984 with a license from DIKTI No. 593/DIKTI/Kep/1993, and the Superior accreditation (LAM-PTKes) based on Decree No. 0178/LAM-PTKes/Akr/Mag/V/2021. This study program is managed by 34 permanent lecturers with doctoral and professor qualifications. Outsourcing programs for the expert staff or practitioners recruitment have also been developed to improve the quality of the teaching and learning process. Master Program in Pharmaceutical Sciences aims to produce graduates who have the competence to:
- Develop the knowledge and technology in the field of pharmaceutical sciences through research to produce innovative and certified projects.
- Solve challenges in knowledge and technology regarding Pharmaceutical Sciences through an inter or multidisciplinary approach.
- Manage research and development that is beneficial to society along with science, including be able to gain national and international recognition.
Academic Degree & Number of Graduates
- Students declared to have passed are entitled to an academic title Master of Pharmacy (M.Farm.).
- As of the end of February 2022, this study program has graduated 409 Masters of Pharmacy from education, government, industry, pharmacy and other institutions
The structured curriculum of MOPS is divided into 7 (seven) specific majors. The MOPS offers the following research interests:
- Pharmaceutical Analysis
- Natural Product Chemistry
- Biomedical Pharmacy
- Pharmacy Policy & Management
- Cosmetics
- Drug Development
- Drug Delivery Systems.
Learning Outcomes (LO)
LO is the required competency to carry out a role established as the graduate profiles. LO is established by referring to the qualification level of the Indonesian National Qualifications Framework and the National Standards for Higher Education. LO consists of Attitude, General Skill, Specific Skill and Knowledge aspects.
A. Attitude
- LO1: Able to realize excellence based on religious morals (excellence with morality), able to work together, and show a responsible attitude to work in their field of expertise independently
- LO2: Able to internalize the spirit of independence, struggle, and entrepreneurship
B. General Skills
- LO3: Able to develop and build logical-critical-systematic-creative thinking and scientific conceptions through scientific research, design creation, or artworks of science and technology that pays attention to and applies humanities values through an interdisciplinary or multidisciplinary approach in the form of a thesis or other equivalent forms
- LO4: Able to develop a pharmaceutical professional performance with analytical acumen in solving pharmaceutical problems and managing research in the pharmaceutical field related to national and global systems and policies, both inter and inter-disciplinary approaches
- LO5: Able to access and review information through an Information and Communication Technology (ICT) system, decide on a specific subject of study, maintain the feasibility of implementing research designs, conduct research, analyze data, conclude research results comprehensively, and create strategic issues based on the study that reflect the latest updates in the field of pharmaceutical sciences, and communicate them in the media and scientific forums at the national and international level through an interdisciplinary or multidisciplinary approach in the form of a thesis or other equivalent forms
- LO6: Able to make decisions in the context of solving problems re lated to science and technology development based on analytical or experimental studies through collaboration with colleagues, colleagues in institutions and research communities at both national and international levels and utilizing research results for the benefit of the user and other communities
C. Special Skills
- LO7: Able to explore natural materials to obtain active ingredients and/or pharmaceutical excipients with due observance of nature conservation
- LO8: Able to carry out drug designs through the synthesis of chemical compounds based on the structure-activity relationship
- LO9: Able to carry out molecular manipulation of substances and develop formulations and manufacturing of pharmaceutical preparations with active pharmaceutical ingredients derived from natural products and synthetic compounds through the manufacture of polymorphs, nanoparticles, solid dispersions
- LO10: Able to develop pharmaceutical management systems and policies related to the referral health care system and the role and function of pharmacists as an integral part of the health care team in order to improve community welfare
- LO11: Able to develop systems for evaluating the bioavailability of drugs in the body, pharmaceutical products circulation permits, and their in-vitro and in-vivo evaluations with specific delivery systems with appropriate analytical methods
- LO12: Able to develop analytical methods to ensure the quality of drugs, cosmetics, foods, and beverages.
D. Knowledge
- LO13: Able to design drug development both from natural products and/or synthetic compounds by considering the biological mimicry system
- LO14: Able to build drug management systems from active pharmaceutical ingredients to finished products that are ready for therapeutic uses
- LO15: Able to plan and organize concepts and procedures for quality assurance and recommendations on pharmaceutical products, which include drugs, cosmetics, foods, and beverages as products and therapeutic goods